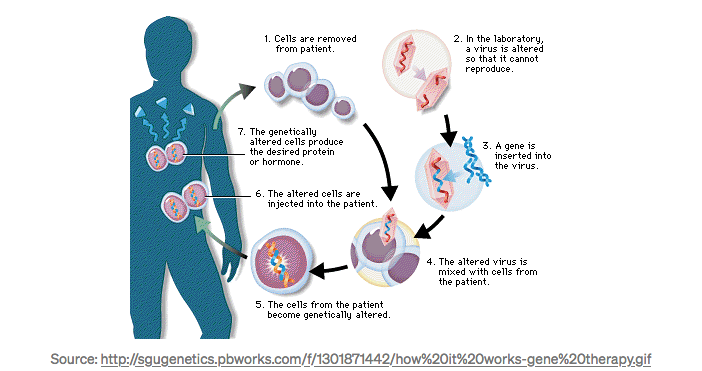
The first gene therapy clinical trial was conducted by William French Anderson and Michael Blaese in 1990 at the NIH Clinical Center. The clinicians used a modified retrovirus that entered cells and integrated into their genomes, but did not reproduce.
The trial enrolled two patients, Ashanti DeSilva and Cynthia Cutshall, suffered from the monogenic genetic disorder adenosine deaminase deficiency (ADA), an autosomal recessive metabolic disorder that causes immunodeficiency. Severe Combined Immunodeficiency, or SCID, occurs in fewer than one in 100,000 live births worldwide. ADA-SCID accounts for about 15% of all cases.
How Does it Work?
Many important questions were left unanswered after that first trial. Since it was a Phase 1 trial (designed to study safety), we don’t know how well the therapy actually worked. During the treatment, both patients remained on PEG-ADA, the standard enzyme replacement therapy. No conclusive results emerged, regarding long-term efficacy.
Twenty-seven years later, on August 30, 2017, the U.S. FDA approved the first ever gene therapy for commercial use. The therapy, called Kymriah, is a chimeric antigen receptor T cell (CAR-T) cell therapy, developed by Novartis. Kymriah was approved for children and young adults with B-cell ALL (an aggressive form of leukemia) that is refractory to standard treatment or has relapsed, at least, twice.
On October 13, 2017, a FDA Advisory Committee voted 16–0 to recommend approval of Spark Therapeutics’ Luxturna. This is a potential one-time, gene therapy for the treatment of vision loss due to confirmed biallelic RPE65-mediated inherited retinal dystrophies, a group of rare blinding conditions caused by one of more than 220 different genes.
Later that month, on October 18, 2017, a second CAR-T therapy, called Yescarta, was approved for the treatment of adult patients with relapsed or refractory large B-cell lymphoma.
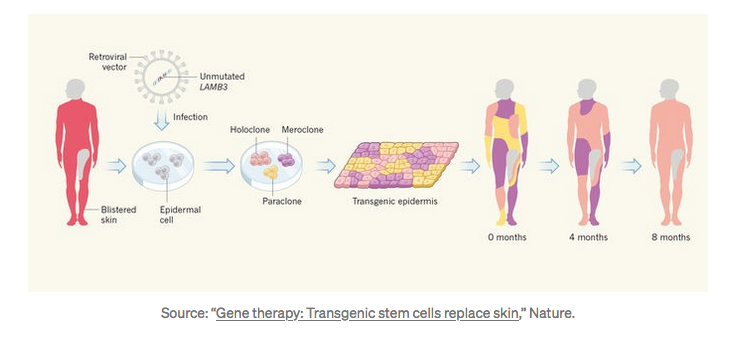
In a truly astonishing breakthrough, Italian researchers were able to almost entirely reconstruct the skin of a seven-year-old boy afflicted with Junctional epidermolysis bullosa, an extremely rare genetic disease — and they used gene therapy to do it.
A medical triumph — saving the life of a 7-year-old boy — using gene therapy and stem cells.
These advances represent critical milestones in the fields of cell and gene therapy. We asked two experts to take a moment to reflect on the tremendous progress they've witnessed, the state-of-the-art, and the challenges that lie along the road to bringing gene therapy to millions of patients.
Q&A with the Experts:
Andre Watson: Founder & CEO, Ligandal, Inc.
Timothy Day: Co-founder & CSO, DNALite Therapeutics
1. BV: How would you describe the industry today?
Andre Watson: The field of biotechnology, and in particular gene therapy, has made strides of advancements over the last two decades. RNA, DNA, CRISPR, other gene editing and therapy tools, as well as delivery systems and antibody-drug conjugate approaches, have steadily advanced into the clinic. The publication rate around each of these categories is skyrocketing exponentially, and technologies are converging.
However, gene therapy approaches that have seen the greatest traction are typically clunky, outdated technologies that lack precision bioengineering. The biggest limitation in the field remains delivery, as the most precise genetic instructions are also the most delicate payloads. A lack of a centralized delivery infrastructure coupled with a drug/gene payload-centric model stymies innovation and progress.
Tim Day: Gene therapy is on an exponential growth curve. Major setbacks in the late 1990’s hung like a dark cloud over the field for the past two decades but doctors, scientists, and entrepreneurs have been hard at work building safer, more efficacious, and targeted gene therapy treatments. We are beginning to see initial success in very difficult to treat diseases and this will facilitate additional treatments.
2. BV: Is the excitement surrounding the space warranted?
Andre Watson: Very much so. The FDA has just recommended the first CAR-T therapy for approval, there are more than 80 gene editing immunotherapy clinical trials underway worldwide, and the first patient has been cured of sickle cell disease through a gene editing approach. While there are still limitations, typically as relate to limited physiological locations that can be reliably delivered to, the field will continue to progress rapidly. Soon, the gap between discovery and translation will narrow even further.
Tim Day: I think the excitement around the space is warranted. Gene therapy has long held the promise of treating intractable diseases and that promise is finally being fulfilled especially for diseases like blood-based cancers, retinal degenerations, liver diseases and skin diseases. The FDA has finally approved the first gene therapy treatment in the U.S. and multiple companies are poised to receive approval as well.
3. BV: In your view, what challenges/roadblocks lie ahead?
Andre Watson: Delivery really is the largest limitation. While we have the precise molecular tools to upregulate, downregulate, delete and insert genes, these tools cannot be reliably delivered outside of liver and certain leaky tumor applications. Additionally, controlling immunogenicity with existing viral therapies is a major limitation, since repeat administrations are not practical with most current approaches. Furthermore, many gene editing approaches involve ablating the bone marrow or immunological system prior to introducing modified cells, which makes many therapies highly invasive and disruptive to quality of life.
Tim Day: Major challenges include delivery of genetic payloads, scaling treatments to reach a large number of patients, and moving beyond monogenic targets to treating more complex genetic diseases. I strongly believe that with enough resources and talent working on these problems we can significantly increase the impact of the gene therapy field.
4. BV: Paint us a vision of the future. How do you envision the industry evolving over the next 5 years? 10 years?
Andre Watson: The industry will evolve such that we will be able to define the precise physiological location (organ, tissue and cell type) as well as genetic instructions that we desire to deliver to that location. After platform technologies for a set of applications are demonstrated, translation of these platforms to other applications will happen with non-linear speed. It will take roughly a decade for this sort of rapid translation to become practical due to FDA timelines, but breakthrough and rare disease applications will push the needle on what we believe to be currently possible. Medicine will become truly personalized, rapid, and extremely precise.
Tim Day: In the next 5-years we will finally see FDA approval of a number of diseases including Leber’s congenital amaurosis, epidermolysis bulosa, and Duchenne muscular dystrophy. Physicians will no longer tell patients who suffer from a number of devastating diseases that there is nothing that can be done but offer a treatment that has meaningful clinical outcomes. In the next 10 years, we will see the rise of personalized medicine and genome editing approaches, such as CRISPR/Cas. Patients will be given a treatment that works best for their particular mutation instead of a broad strokes approach.
5. BV: What are you working on? Explain the significance to the industry’s bigger picture.
Andre Watson: Ligandal is developing delivery systems for genes, gene editing tools, and other molecular payloads. The thesis of our development is to separate targeting (ligands) from function (payloads), and to enable deterministic programming of biological systems to this end. We are building a universal delivery platform to create value across the entire industry and replace the need for viruses.
Tim Day: I am the co-founder of DNALite Therapeutics an early-stage gene therapy company focused on delivering genetic cargo to tissues protected by mucus barriers. We are initially developing a first-of-its-kind oral gene therapy treatment for Familial adenomatous polyposis, which is a genetic disease that results in a near 100% chance of colon cancer by age 40. The significance is that we are increasing the scope of tissues, like the gastrointestinal tract, lungs, and cervix that can be successfully targeted by gene delivery vehicles allowing us to reach a number of critical unmet needs.
6. BV: Anything else you would like to discuss that’s not addressed above?
Tim Day: As our understanding of biology increases and the cost of doing biology decreases (especially genetics), I would encourage interested individuals to jump into the biotech field and help bring the next generation of medicine to the world. A better future is not inevitable. It must be built.
Since this interview, many new approaches to gene therapy have come to light. Therapy vehicles have evolved from viral vectors or injected DNA to non-viral, peptide encapsulated constructs. The newer vehicles permit greater specificity in tissue targeting and are less likely to provoke an immune response. Gene replacement and alteration are increasingly the province of the extremely precise CRISPR/Cas9 system.
Summary
Gene therapy has reached a critical inflection point and the future is very bright: for patients, entrepreneurs, and investors. We couldn’t agree more with Tim’s closing sentiment. At Bioverge, we’re here to support the next generation of scientists and entrepreneurs who are building the future.
-The Bioverge Team
Disclosures:
Ligandal is a Bioverge portfolio company.